Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sato, Takumi; Nagae, Yuji; Kurata, Masaki; Quaini, A.*; Gu neau, C.*
neau, C.*
CALPHAD; Computer Coupling of Phase Diagrams and Thermochemistry, 79, p.102481_1 - 102481_11, 2022/12
Times Cited Count:0 Percentile:0.01(Thermodynamics) Pu
Pu )O
)O at 1773 and 1873 K, and its analysis based on point defect chemistry
at 1773 and 1873 K, and its analysis based on point defect chemistryKato, Masato; Nakamichi, Shinya; Takeuchi, Kentaro; Sunaoshi, Takeo*
CALPHAD; Computer Coupling of Phase Diagrams and Thermochemistry, 35(4), p.623 - 626, 2011/12
Times Cited Count:12 Percentile:54.56(Thermodynamics)Uranium and plutonium mixed oxide (MOX) has been used as fuels of fast reactors. The MOX having fluorite structure is an oxygen nonstoichiometric compound which is stable in hyper- and hypo-stoichiometric composition range. The stoichiometry of MOX significantly affects their properties. So, it is essential to know the relation between stoichiometry and oxygen potential to develop MOX fuels. In this work, the oxygen potentials of (U Pu
Pu )O
)O were measured at high temperatures of 1773, and 1873K. The measurements were carried out by gas equilibrium method using thermo-gravimetry. Th The oxygen partial pressure was adjusted by controlling the ratio of H
were measured at high temperatures of 1773, and 1873K. The measurements were carried out by gas equilibrium method using thermo-gravimetry. Th The oxygen partial pressure was adjusted by controlling the ratio of H /H
/H O in the flowing gas atmosphere, and the oxygen potential was determined. The oxygen potentials were determined as functions of O/M ratio, and temperature. The data at stoichiometric composition were estimated to be -311kJ/mol and -299kJ/mol at 1773K, and 1873K based on point defect model.
O in the flowing gas atmosphere, and the oxygen potential was determined. The oxygen potentials were determined as functions of O/M ratio, and temperature. The data at stoichiometric composition were estimated to be -311kJ/mol and -299kJ/mol at 1773K, and 1873K based on point defect model.
Nakamichi, Shinya; Kato, Masato; Tamura, Tetsuya*
CALPHAD; Computer Coupling of Phase Diagrams and Thermochemistry, 35(4), p.648 - 651, 2011/12
Times Cited Count:11 Percentile:52.23(Thermodynamics)Japan Atomic Energy Agency has developed homogeneous MOX fuels containing minor actinides (MAs) such as Am and Np elements. In this work, particular attention is paid to the influence of small MA addition on oxygen potential of MOX fuels. Oxygen potentials of (Am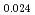 Pu
Pu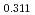 U
U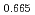 )O
)O and (Am
and (Am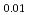 Np
Np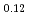 Pu
Pu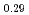 U
U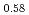 )O
)O in the near stoichiometric region were measured at 1473, 1573 and 1623K by thermogravimetry. (Am
in the near stoichiometric region were measured at 1473, 1573 and 1623K by thermogravimetry. (Am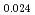 Pu
Pu U
U )O
)O and (Am
and (Am Np
Np Pu
Pu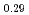 U
U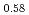 )O
)O were slightly higher than those of (Pu
were slightly higher than those of (Pu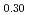 U
U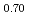 )O
)O . From this work, it was found that oxygen potential of MOX is slightly increased by small Am addition and influence of Np addition on oxygen potential is not significant.
. From this work, it was found that oxygen potential of MOX is slightly increased by small Am addition and influence of Np addition on oxygen potential is not significant.